Phase diagrams for binary salt solutions
Phase diagrams for several binary salt solutions are shown below. Click on the tabs to see solubility diagrams for:
- Sodium chloride – water
- Calcium chloride – water
- Magnesium nitrate – water
- Magnesium sulfate – water
- Tri-sodium phosphate – water
- Di-sodium phosphate – water
- Mono-sodium phosphate – water
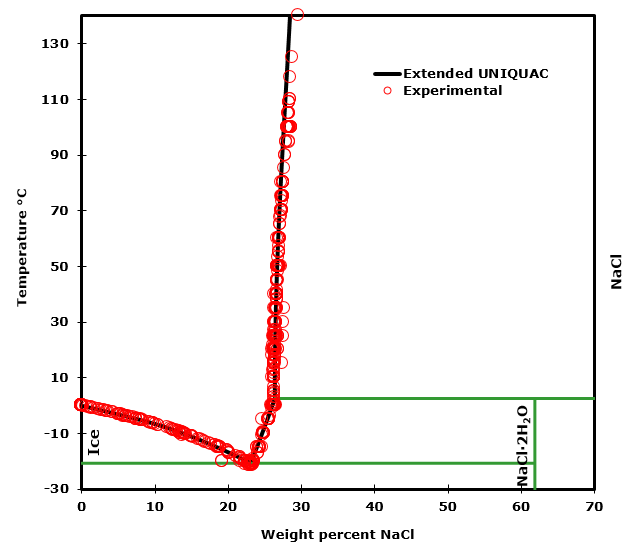
The sodium chloride – water system
In the sodium chloride – water system, one eutectic point and one peritectic point is found. These points are shown in the phase diagram to the right.
The eutectic point is at -21°C.
The peritectic point is at 0.1°C.
The eutectic point is the cryohydratic point, where ice and NaCl·2H2O (hydrohalite) both are in equilibrium with the same saturated solution. This point is at the lowest temperature where a liquid sodium chloride solution can exist.
The peritectic point at 0.1 °C marks the transition between hydrohalite and anhydrous sodium chloride as the stable solid phase in equilibrium with a saturated sodium chloride solution. If heated, solid hydrohalite will at this temperature be transformed into anhydrous sodium chloride (NaCl) and a saturated solution of sodium chloride.
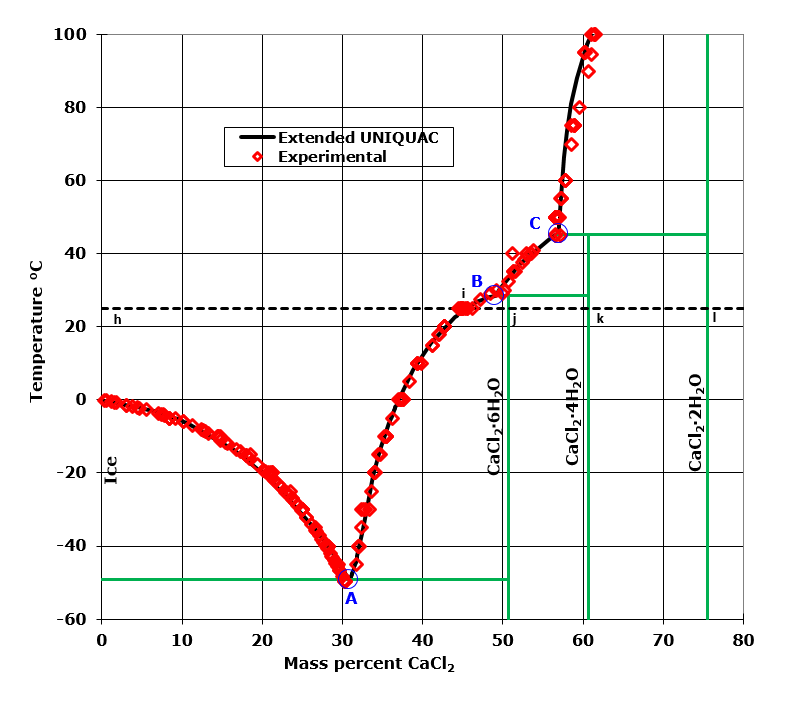
The Calcium Chloride – water system
Pure water freezes to ice at 0°C. If CaCl2 or another solute is added to water, the freezing point of the mixture will be lower than 0°C. This phenomenon is called a freezing point depression. It can be explained from changes in chemical potentials.
A solution containing 31 mass % CaCl2 has the lowest freezing point of any CaCl2 solution (about -50°C) . This solution is called an eutectic solution. The point (A) in the diagram marking the freezing point of this solution is an eutectic point, also called a cryohydric or cryohydratic point. At the freezing point of an eutectic CaCl2 solution, the solution is in equilibrium with two solid phases: ice and CaCl2·6H2O. Counting also the vapor phase, a total of four phases are in equilibrium in this binary system. The eutectic point therefore constitutes an invariant point. An invariant point has no degrees of freedom – one specific temperature, pressure, and concentration is required.
The curve between the points A and B marks compositions of solutions that are saturated with the hexahydrate, CaCl2·6H2O.
The curve between B and C marks compositions of solutions that are saturated with the tetrahydrate, CaCl2·4H2O. The dihydrate CaCl2·2H2O is in equilibrium with saturated solutions at temperatures higher than that marked by point C. At even higher temperatures, saturated solutions are in equilibrium with the anhydrate, CaCl2, (not shown in the diagram).
The points B and C are peritectic points. In a peritectic point, a solid phase changes upon heating into a liquid in equilibrium with another solid phase. CaCl2·4H2O consists of 60.6 mass percent CaCl2. This composition is marked with a vertical green line in the figure. If CaCl2·4H2O is heated to 45.3°C it will decompose into a liquid in equilibrium with CaCl2·2H2O. The temperature at which this happens is marked with a horizontal green line. The two points B and C represent solutions in equilibrium with two solid phases and a gas phase and therefore constitute invariant points.
The 25°C isotherm is marked with a dashed, black line in the diagram. Between h and i the mixture is liquid. No solid precipitates. Point i marks the interception between the dashed line and the solubility curve. In this point, the solution is saturated with CaCl2·6H2O, but no precipitate has formed. Between i and j, increasing amounts of hexahydrate, CaCl2·6H2O is precipitating.
Some liquid with a composition at i remains in equilibrium with this solid. In point j, the composition of the solution corresponds to the composition of CaCl2·6H2O. At this point, there is no liquid but only solid CaCl2·6H2O. Between j and k, mixtures consist of CaCl2·6H2O (s) and CaCl2·4H2O (s), no liquid is present. Between k and l, mixtures consist of CaCl2·4H2O (s) and CaCl2·2H2O (s), no liquid is present. Mixtures with CaCl2 concentrations higher than that marked by l consist of CaCl2·2H2O (s) and CaCl2(s).
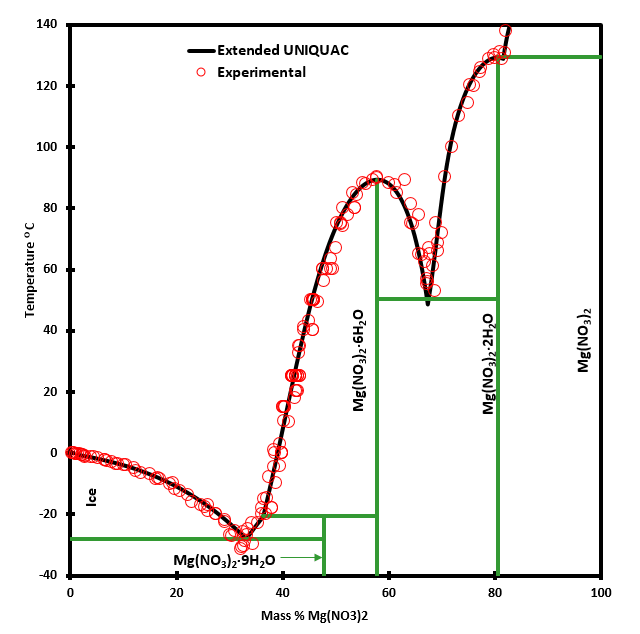
The magnesium nitrate – water system
The magnesium nitrate – water system exhibits a particular solubility behavior: multiple solubilities at the same temperature. This kind of behavior is encountered in many aqueous salt systems. It is annoying for the person designing processes in such systems.
Between 50 and 90°C the solubility of magnesium nitrate can be one of three values, as it appears from the phase diagram below. The figure also shows that the Extended UNIQUAC model is able to reproduce this type of solubility behavior quite accurately. Three eutectic points and one peritectic point are found in this binary system.
The eutectic point where ice and magnesium nitrate nona-hydrate precipitate simultaneously is at -27°C and 33 % Mg(NO3)2. This is the cryohydratic point. A peritectic point appears at -21°C and 36 % Mg(NO3)2 marking the transition between the nona-hydrate and the hexa-hydrate of magnesium nitrate.
The hexahydrate and the dihydrate of magnesium nitrate form a simple eutectic system with a eutectic point at 50°C and 67 % Mg(NO3)2. Finally magnesium nitrate di-hydrate and anhydrous magnesium nitrate form a simple eutectic system with an eutectic point at 129°C and 82 % Mg(NO3)2.
The fact that the transition at 129°C is a eutectic rather than a peritectic point has been documented in a number of experiments as for example the measurements carried out by Ewing, W. W., Brandner, J. D., Slichter, C. B., Griesinger, W. K. “The temperature-composition relations of the binary system magnesium nitrate-water”, J. Am. Chem. Soc. 55(1933)4822-24 https://doi.org/10.1021/ja01339a013.
The magnesium sulfate – water system
In the magnesium sulfate – water system, one eutectic point and four peritectic points are found. The Eutectic point and three of the peritectic points are shown in the figure below.
At temperatures above 73°C, the solubility of magnesium sulfate decreases with increasing temperature. This solubility behavior up to 140°C is described very well by the Extended UNIQUAC model as shown in the figure below. At higher temperatures, there is some discrepancy between model calculation and experimental data, but the decreasing trend continues.
In the phase diagram, the solid phase at temperatures between the eutectic point and -2°C is marked as MgSO4·12H2O. New research shows that this solid is actually Meridianiite, MgSO4·11H2O. This mineral was found in a frozen pond in British Colombia, Canada by Peterson et al. (R.C. Peterson, W. Nelson, B. Madu, and H.F. Shurvell, Meridianiite: A new mineral species observed on Earth and predicted to exist on Mars, American Mineralogist; October 2007; v. 92; no. 10; p. 1756-1759; https://doi.org/10.2138/am.2007.2668).
The other solid phases marked in the phase diagram are:
- Epsomite, MgSO4·7H2O – stable solid phase in binary aqueous solution at temperatures between -2 and 52°C
- Hexahydrite, MgSO4·6H2O – stable solid phase in binary aqueous solution at temperatures between 52 and 73°C
- Kieserite, MgSO4·H2O – stable solid phase in binary aqueous solution at temperatures to at least 194°C

Phase diagram for the MgSO4 – H2O system. At temperatures above 73°, the solubility of MgSO4 is decreasing. Experimental data are marked with red circles. The calculated solubility curve is marked with a black line.
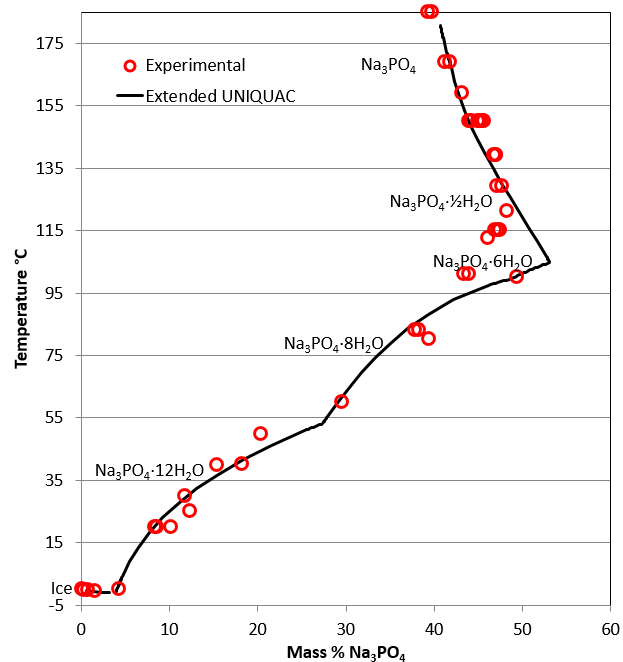
The trisodium phosphate – water system
In the phase diagram for the Na3PO4, trisodium phosphate – water system shown to the right, one eutectic point and four peritectic points exist. The eutectic point is the cryohydratic point, where ice and Na3PO4·12H2O both are in equilibrium with the same liquid.
The phase diagram consists of six different branches including ice, four hydrated forms and anhydrous trisodium phosphate.
Four peritectic points mark the transitions
- Na3PO4·12H2O ↔ Na3PO4·8H2O at around 54°C
- Na3PO4·8H2O ↔ Na3PO4·6H2O at around 100°C
- Na3PO4·6H2O ↔ Na3PO4·½H2O at around 105°C
- Na3PO4·½H2O ↔ Na3PO4 at around 165°C.
Above this temperature, the anhydrous form Na3PO4 is the stable phase. The solubility of the hemi-hydrate and the anhydrous form decrease with increasing temperature. There is some discrepancy between experimental solubility data from different sources and between model calculations and experimental data, especially in the temperature range from 100 to 120°C.
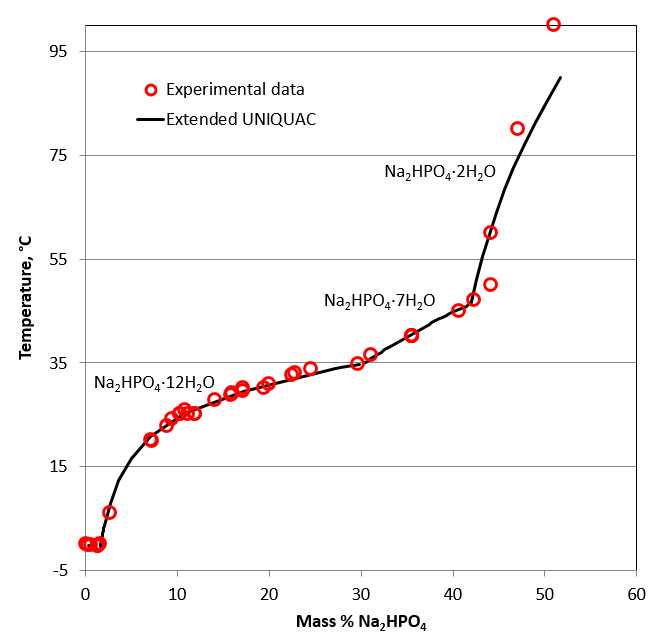
The disodium phosphate – water system
Next, the phase diagram for the disodium phosphate – water system is shown. The stable phases at the eutectic point are ice and disodium phosphate dodecahydrate, Na2HPO4·12H2O.
This phase diagram consists of four different branches including ice, dodeca-, hepta- and di-hydrate of disodium phosphate.
Two peritectic points mark the following transitions:
- Na2HPO4·12H2O ↔ Na2HPO4·7H2O at around 35°C
- Na2HPO4·7H2O ↔ Na2HPO4·2H2O at around 46°C
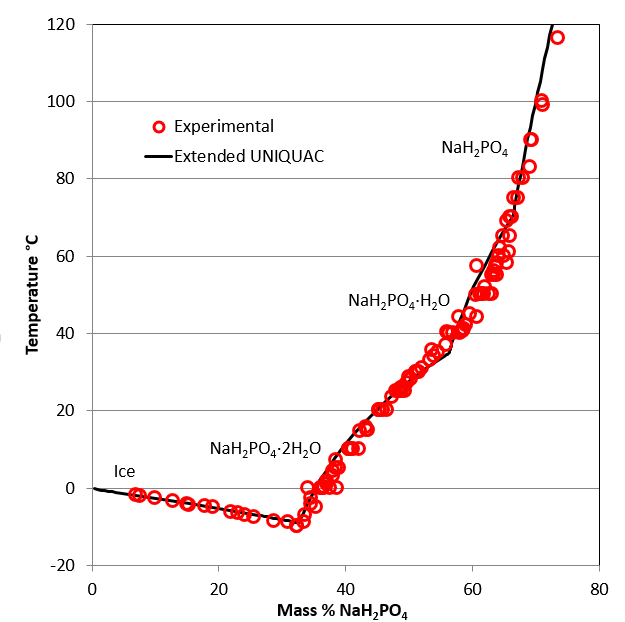
The monosodium phosphate – water system
In the monosodium phosphate – water system, three solid phases besides ice can be in equilibrium with saturated solutions: a dihydrate, monohydrate and the anhydrous form. The phase digram is shown at last.
The stable phases at the eutectic point are ice and monosodium phosphate, dihydrate, NaH2PO4·2H2O.
The phase diagram indicates two peritectic points:
- NaH2PO4·2H2O ↔ NaH2PO4·H2O at around 35°C
- NaH2PO4·H2O ↔ NaH2PO4 at around 71°C
In all three figures below, the circles mark experimental data from the open literature. Lines mark the solubility calculated with the Extended UNIQUAC model.
Phase diagram for the trisodium phosphate – water system. The phase diagram consists of six different branches, including ice.
Phase diagram for the trisodium phosphate – water system. Experimental data are marked with red circles. the calculated equilibrium curve is marked with a black line.
Phase diagram for the monosodium phosphate – water system up to 120°C